Site-specific protein labeling with infrared probes

Although IR methods are straightforward to employ with small molecules, their application to proteins is complicated by spectral congestion. We overcome this challenge by incorporation of IR probes with frequencies between 1900-2500 cm-1, a region free of intrinsic protein absorptions. Such an IR probe can provide a reporter of the microenvironment at a specific site anywhere in a protein. We take advantage of a number of suitable IR probes.
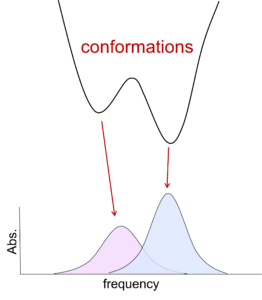
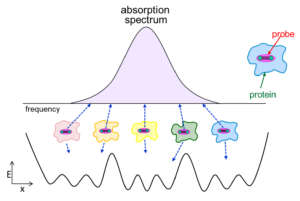
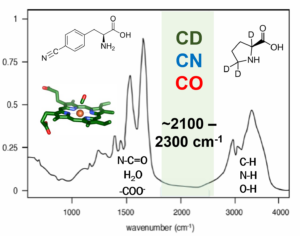
For hemeproteins, such as cytochrome P450s, heme-bound CO provides an intense vibrational band that shows high sensitivity to local electrostatics, making it an excellent probe of the local environment surrounding the heme center. To enable characterization of any site in any protein, we incorporate IR probes on amino acid side chains. Carbon deuterium bonds are strictly non-perturbative IR probes that directly probe the protein itself. Cyano groups provide more intense signals for nonlinear spectroscopy. We achieve site-specific incorporation of the IR probes via a variety of routes, including expression in defined media, the in vivo amber suppression methodology, peptide synthesis and native chemical/expressed protein ligation, and the introduction of thiocyanate groups via chemical modification of Cys residues. By characterization of proteins via multiple IR probes introduced at different locations, we generate spatially complete information about the local environments and dynamics, which enables us to explore how they are involved in protein function.