Functional dynamics of Cytochrome P450s

P450s are oxidases which, in addition to their central role in drug metabolism, hydroxylate unactivated carbon centers with high regio- and enantioselectivity, making them a subject of considerable interest. P450 homologs can vary greatly in their substrate specificity, and with a given substrate, their regio- and enantio-selectivity. As P450s have similar structures and catalytic mechanisms, protein flexibility is sometimes evoked to explain the variation in P450 activity, however we lack a rigorous molecular-level understanding of how conformational heterogeneity and dynamics are involved. To address this question, we are applying our IR-based approach to measure P450 conformations and dynamics to unravel their contribution to the activity of these enzymes, including their catalytic selectivity and substrate specificity.
Photosynthetic electron transfer

Unlike electron transfer between small molecules, biological electron transfer occurs between redox centers embedded within protein environments that are structurally and electrostatically heterogeneous. Furthermore, inter-protein electron transfer is often gated by transient protein-protein interactions, which is thought to facilitate the efficient but controlled transfer of electrons. These features have challenged rigorous characterization, and thus our understanding of biological electron transfer remains incomplete. Our research program aims to overcome these difficulties and delineate the mechanisms by which proteins mediate electron transfer, specifically focusing on electron transfer involving plastocyanin and cytochrome f, two key proteins of the photosynthetic apparatus. Towards this end, we have used nonperturbative vibrational probes to directly monitor the Cu center of plastocyanin.
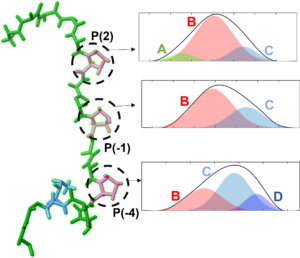
Molecular recognition and proline conformational heterogeneity
Proline can take on multiple conformations on its own and in peptide sequences, and understanding the vibrational signatures of each state can aid in understanding unique proline-rich sequences. SH3 domains bind to such proline-rich sequences, and play a significant role in recognition of appropriate binding partners within a complex cellular environment. A potential determinant of protein specificity is the degree of conformational heterogeneity and dynamics. We are testing this and other possible mechanisms underlying specificity by elucidation of the conformations and dynamics involved in protein molecular recognition with unprecedented detail. We also aim to use our sensitivity to the conformational states of proline itself to study proline-rich biological polymers.